DUAKLIR PRESSAIR Demonstrated Efficacy in 3 Trials
Significant improvement in lung function (peak) FEV1 vs mono-components and tiotropium at Week 242,3,9
DUAKLIR PRESSAIR vs mono-components and tiotropium (AMPLIFY trial)†
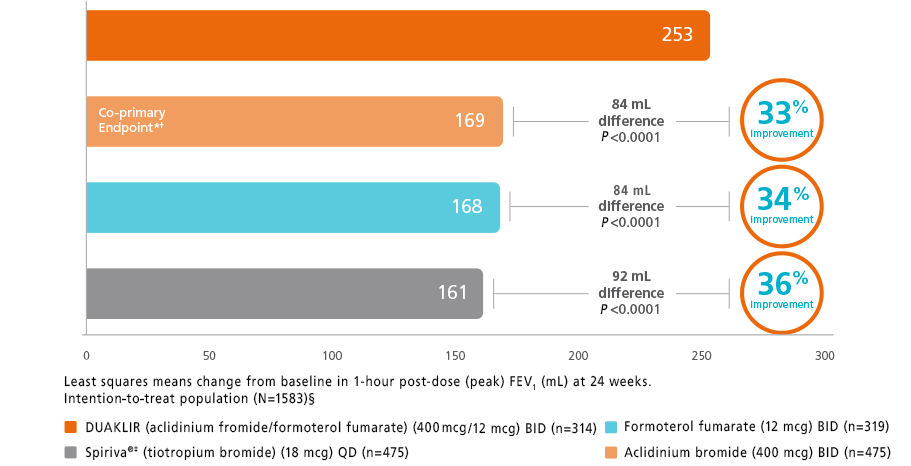
*The co-primary endpoint was change from baseline in 1-hour morning post-dose (peak) FEV1 at Week 24 for DUAKLIR PRESSAIR 253 mL vs aclidinium 169 mL (P<0.0001).9
†The second co-primary endpoint was change from baseline in morning pre-dose (trough) FEV1 at Week 24 for DUAKLIR 80 mL vs formoterol 25 mL (P<0.001).9
‡Spiriva® is a registered trademark of Boehringer Ingelheim International GmbH.
§Of 1,594 patients randomized, 1,583 were
included in the ITT and
safety populations, 1,403 in the per protocol population, and 1,356 (85.1%)
remained on study treatment and completed the study.
FEV1 = forced expiratory volume in 1 second;
BID = twice daily;
QD = once daily;
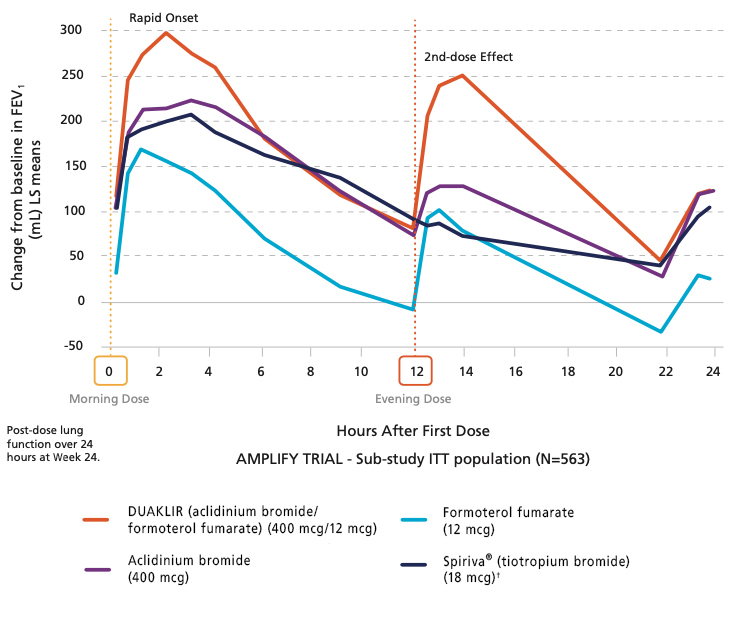
24-Hour Control—Improving Breathing Both Day and Night
A serial spirometry sub-study of AMPLIFY, which evaluated the bronchodilation time-profile over 24 hours post-dose at Week 242,3,*
LS = least squares;
FEV1 = forced expiratory
volume in 1
second;
ITT = intention-to-treat
*Aclidinium bromide/formoterol fumarate 400 mcg/12 mcg twice daily compared to its component parts (aclidinium bromide 400 mcg twice daily or formoterol fumarate 12 mcg twice daily) and once-daily tiotropium 18 mcg.
†SPIRIVA® is a registered trademark of Boehringer Ingelheim International GmbH.
At Week 24, lung function improvement (peak) FEV1 was significantly greater with DUAKLIR vs either monotherapy2,5,10
DUAKLIR vs mono-components (AUGMENT trial)†
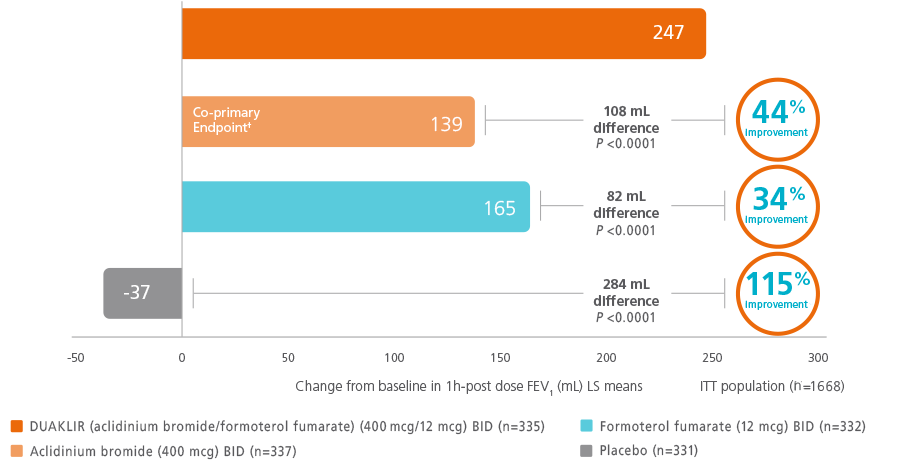
†The co-primary endpoint was change from
baseline in
1-hour
morning
post-dose FEV1 at Week 24 for DUAKLIR 253 PRESSAIR mL vs aclidinium 169 mL (P
<0.0001) and change from baseline to Week 24 in morning pre-dose (trough)
FEV1 DUAKLIR
vs formoterol.10
ITT = intention-to-treat;
LS = least squares;
FEV1 = forced expiratory
volume in 1 second;
BID = twice daily
Lung function improvement (peak) FEV1 was significantly greater with DUAKLIR vs either monotherapy or placebo at Week 242,11,13
DUAKLIR vs mono-components (ACLIFORM trial)†
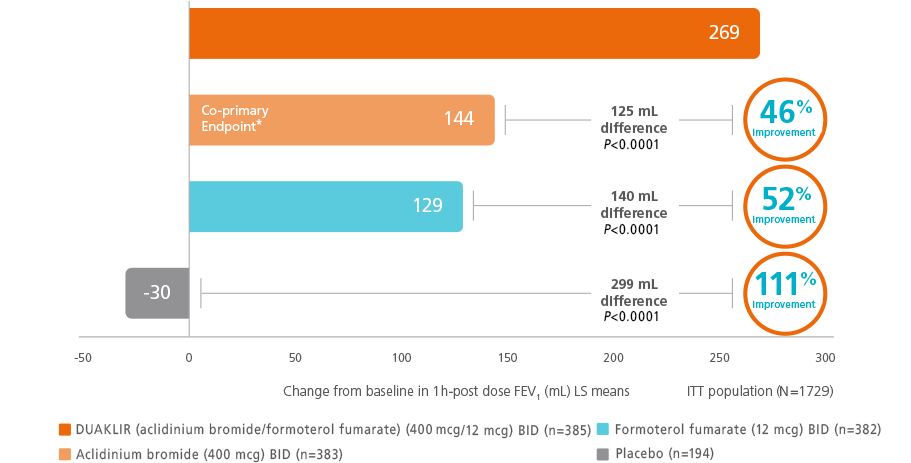
†The co-primary endpoint was change from
baseline in 1-hour
morning
post-dose FEV1 at Week 24 for DUAKLIR PRESSAIR 253 mL vs aclidinium 169 mL.11
ITT = intention-to-treat;
LS = least squares;
FEV1 = forced expiratory volume
in 1 second;
BID = twice daily
Rapid Onset of Action—as Quickly as 5 Minutes2,3,5,8,*,†,‡
DUAKLIR PRESSAIR provides patients with better breathing in as fast as 5 minutes.
*In two Phase 3 studies (ACLIFORM-COPD and AUGMENT-COPD), DUAKLIR PRESSAIR displayed an increase in FEV1 compared to placebo of 0.108 L (95% CI; 0.089, 0.127) and 0.128 L (95% CI; 0.111, 0.145) within 5 minutes after the first dose, respectively.2
†Limitations of use: Not indicated for the relief of acute bronchospasm or for the treatment of asthma.
‡The co-primary endpoints were change from baseline at Week 24 in 1-hour morning post-dose (peak) FEV1 versus aclidinium 400 mcg and morning pre-dose (trough) FEV1 versus formoterol 12 mcg.
FEV1 = forced expiratory volume in 1 second
Sustained Improvement in Lung Function (peak) FEV1
In the AMPLIFY trial, the effect of DUAKLIR was sustained over the 24-week treatment period and FEV1* was statistically superior to either of its individual components and tiotropium at all timepoints2,3,9
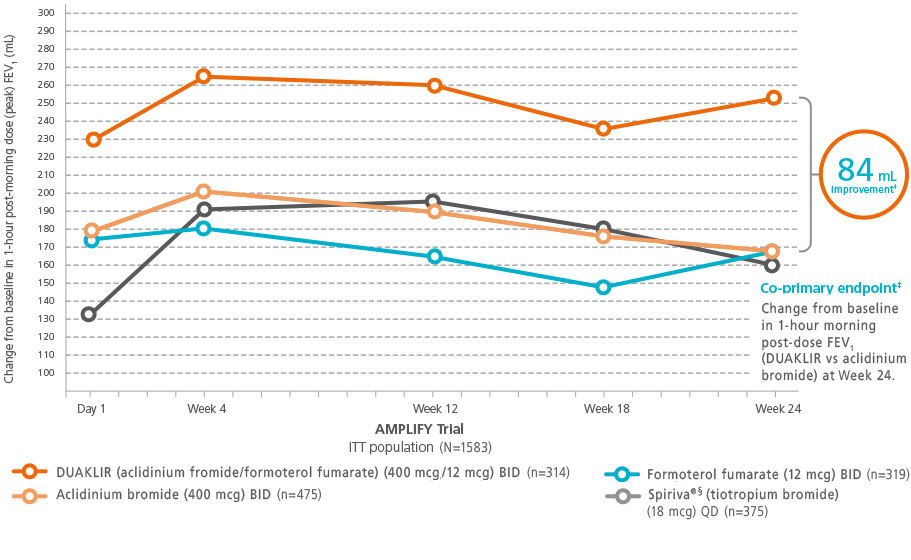
*FEV1 obtained 1-hour post-morning dose.
†P<0.001
‡The second co-primary endpoint was change from
baseline in morning pre-dose (trough) FEV1 at Week 24 for
DUAKLIR 80 mL vs
formoterol
25 mL (P<0.001).
§Spiriva® is a registered trademark
of Boehringer Ingelheim International GmbH.
ITT = intention-to-treat;
FEV1 =
forced
expiratory volume in 1 second;
BID = twice daily;
QD = once daily
DUAKLIR PRESSAIR Safety Profile
Adverse reactions occurring in the DUAKLIR PRESSAIR group at a frequency of ≥3% and exceeding placebo2,*
| empty | Treatment | |||
|---|---|---|---|---|
| Adverse Reactions Preferred Term |
DUAKLIR PRESSAIR (n=720) |
Aclidinium (n=722) |
Formoterol (n=716) |
Placebo (n=526) |
| Upper respiratory tract infection† | 8.9% | 7.6% | 8.9% | 6.3% |
| Headache | 6.3% | 6.6% | 7.7% | 5.1% |
| Back pain | 3.8% | 3.3% | 3.5% | 3.4% |
*Pooled safety analysis from two 24-week, placebo-controlled studies in adult patients with moderate-to-severe COPD.
†Includes viral upper respiratory tract infection and upper respiratory tract infection.
- Other adverse reactions reported in clinical studies with an incidence of 1% to 3% with DUAKLIR PRESSAIR and more common than with placebo were cough, sinusitis, influenza, tooth abscess, insomnia, dizziness, dry mouth, oropharyngeal pain, muscle spasms, musculoskeletal pain, arthralgia, pain in extremity, urinary tract infection, and blood creatine phosphokinase increased
- The adverse events reported in the 24-week active-controlled trial were consistent with those observed in 24-week placebo-controlled trials
Long-term Safety
DUAKLIR PRESSAIR 400 mcg/12 mcg twice daily was assessed in a 28-week safety extension trial in subjects who successfully completed trial 2 (AUGMENT-COPD) for a total treatment period of up to 52 weeks. The adverse reactions reported in the long-term safety trial were consistent with those observed in the 24-week, placebo-controlled trials.
