Clear the Pathway to What's Possible With DUAKLIR
The twice-daily dosing of DUAKLIR® PRESSAIR® provides your patients with improved lung function in the morning and evening2, which may help them rediscover their ability to do normal, everyday activities, all day.
Your Patients Don’t Have to Settle for a Diminished Quality of Life Due to COPD
The St George's Respiratory Questionnaire (SGRQ) is a patient-reported, respiratory disease-specific instrument designed to measure4:
![]()
More Patients
Achieved clinically meaningful improvement* in health-related quality of life for patients with stable COPD taking DUAKLIR vs placebo.5
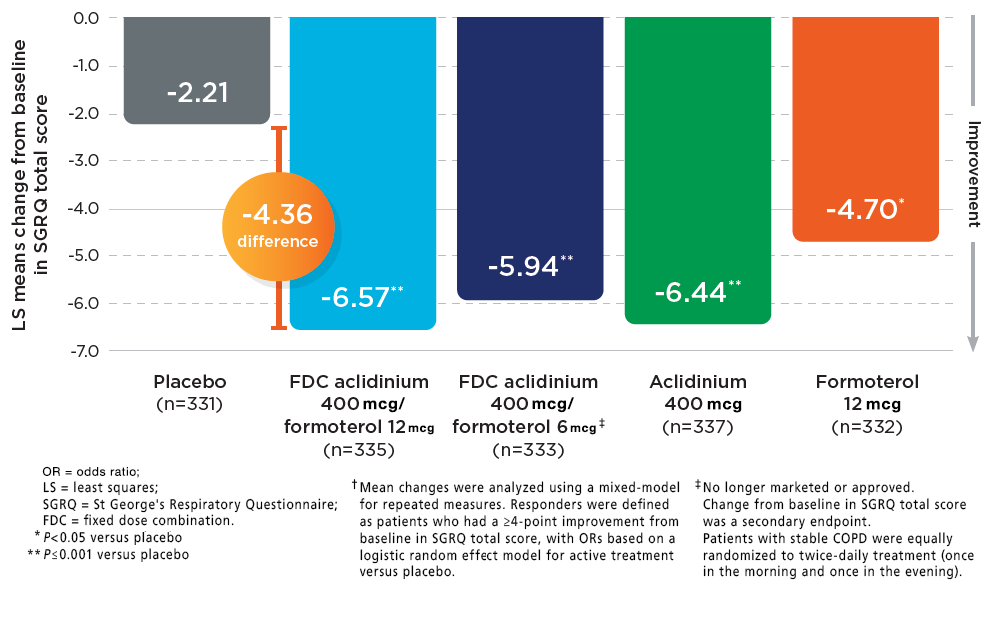
In one Phase 3 clinical trial (AMPLIFY COPD), the SGRQ responder† rate at Week 24 for DUAKLIR was 58% compared to 39% for placebo, with an odds ratio (OR) of 2.3 (95% Cl: 1.41, 3.61).2
*Mean changes were analyzed using a mixed-model for
repeated measures. Responders were defined as patients
who had a ≥4-point improvement from baseline in SGRQ
total score, with ORs based on a logistic random effect
model for active treatment versus placebo.
†DUAKLIR vs placebo did not meet statistical
significance in two other Phase 3 clinical trials
(AUGMENT COPD, ACLIFORM COPD).
Cl=confidence
interval.
DUAKLIR PRESSAIR Demonstrated Clinically Meaningful Improvement In Quality Of Life vs Placebo5
MEAN CHANGES FROM BASELINE AT WEEK 24†
 View Study Description
View Study Description

COPD is an Around-the-Clock Symptomatic Disease
Despite regular treatment, over 90% of patients with stable COPD experience symptoms at some point during the day, with the most troublesome times of the day being early morning and nighttime.7
Clinical2,7
Nighttime symptoms or awakenings
Shortness of breath
Chronic cough
Emotional6
Defeated in how COPD has affected their daily lives and the activities they used to do
Helpless as their sphere of activities continues to shrink due to COPD
Dual Bronchodilators for Improved Benefits*
DUAKLIR PRESSAIR combines long-acting aclidinium bromide with formoterol fumarate—providing your patients with powerful dual bronchodilation, taken twice daily for full 24-hour symptom control.2
*Benefits are defined as improvement in lung function with one bronchodilator vs two bronchodilators.
†Limitations of Use: DUAKLIR PRESSAIR is not indicated for the relief of acute bronchospasm or for the treatment of asthma.
